ADC manufacturing

Antibody Drug Conjugates (ADCs) are one of the fastest-growing therapeutic classes in oncology with more than 120 ADCs in active clinical development and around 200 companies actively developing ADCs.
ADCs are complex molecules composed of:
- An antibody: a whole monoclonal antibody (mAb) or an antibody fragment such as a single-chain variable fragment
- A stable chemical linker with labile bonds
- An active cytotoxic payload
By combining the unique targeting capabilities of monoclonal antibodies with the cancer-killing ability of cytotoxic drugs, antibody-drug conjugates allow sensitive discrimination between healthy and diseased tissue.
Therefore, the manufacturing of ADCs requires biological and chemical know-how, as well as expertise in handling highly toxic compounds, which Axplora has mastered for many years.
Our expertise in ADC manufacturing
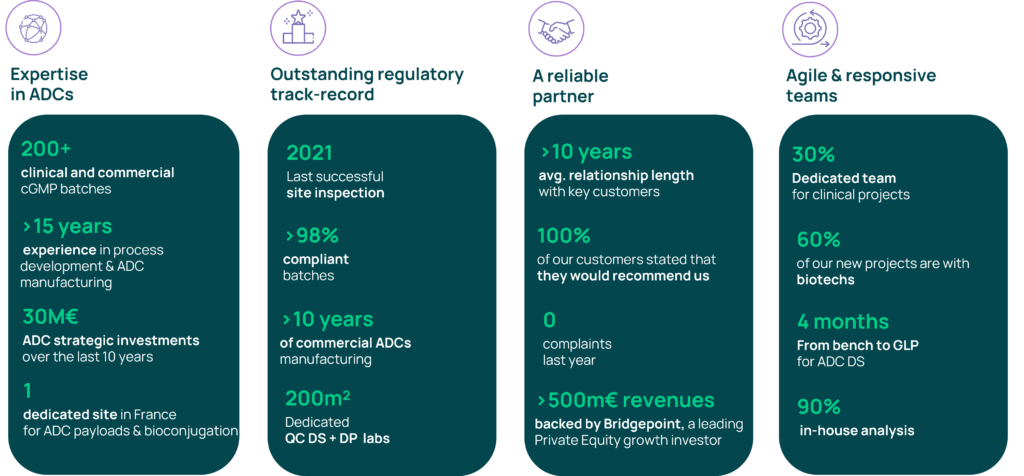
Axplora is respected as a key contract service provider in the ADC arena with more than 15 years of experience. We provide unique services for the development, fast-track, clinical supply, and commercial cGMP manufacture of ADCs including the payload linker and the bioconjugation.
By working with us on the development and manufacturing of your ADCs, you benefit from:
- Phase-adapted, scientifically sound development throughout your ADC drug lifecycle
- Multi-scale bioconjugation manufacturing capacity with short-term availability
- Fast-track bioconjugation services with seamless transition to clinical supply
- One-stop-shop for Drug Substance and Drug Product analytical services
- A 15-year clinical & commercial track-record – 200+ cGMP batches
- A cross-functional and collaborative approach between ADC experts
- Robust processes & analytics for complex molecules
- An excellent regulatory history

ADC manufacturing solutions
Axplora offers a full and flexible range of development, analytical, and manufacturing services for ADCs, whatever the stage of your project:
- Fast track offer for preclinical studies – One ADC sample with RoA
- 1 – 100 mg ADC sample
- Platform analytical methods
- 6 weeks turnaround time
- Phase I-II package – First in human clinical trials
- Process & analytical development
- Phase-adapted validation
- cGMP manufacturing
- Methods validation
- Phase III package – Support to validation
- Process characterization
- ICH analytical validation
- Process validation
- BLA regulatory support
- Commercial package – Lifecycle support
- Continuous improvement
- Product lifecycle management
Our dedicated team can tailor a custom manufacturing package. We will work with you to create a scope of work to complete the project on time and within your specifications.

ADC manufacturing capabilities
To learn more about Axplora’s ADC capabilities, download our brochure.

Center of excellence for ADC manufacturing
Axplora’s Le Mans site, located in France, specializes in ADCs. It offers a long track record in ADC production for payload synthesis & purification and bioconjugation. The site has been successfully inspected by the FDA and the European authorities recently.
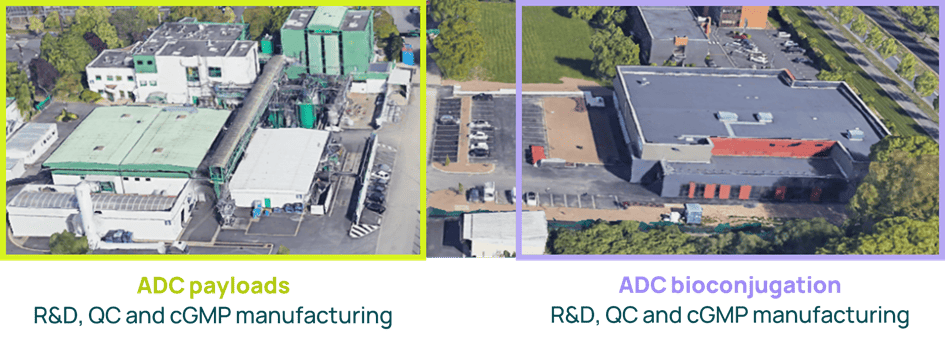
Axplora’s ongoing investment in the Le Mans site demonstrates our commitment to ADCs:
- 30M€ invested over the last ten years
- Pharmaceutical Establishment licensed for the release testing of ADC drug products

