Our Group in numbers
One group. Three stories.
Millions of patients served.
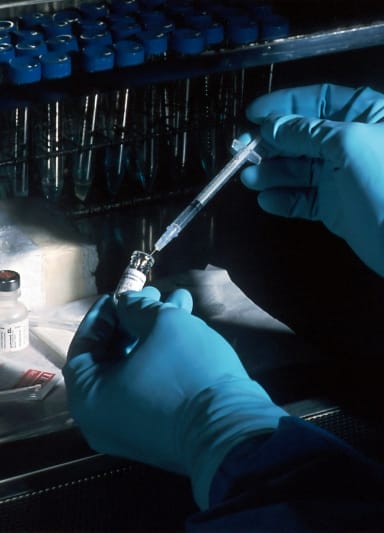
Novasep CDMO strengthens its innovative position in oncology
We invested €8 million in our Le Mans (Fr) site to expand our production capabilities for manufacturing ADCs. Behind every pharma project, there are high-quality services for our customers and cutting-edge drugs for millions of patients worldwide.
Combining strengths to provide existing and innovative treatments for patients
“Axplora possess a unique blend of technical expertise (chromatography and small molecule manufacturing), interpersonal skills, and a dedication to excellence that makes them an exceptional CMO. We are fortunate to have such a strong partnership with the Axplora team!”

“The Axplora team was very open, flexible and efficient in working through project changes.”

Francesco Liotino
Director of Business Development
“I represent the dynamic and innovative team at Axplora. As we gear up for the upcoming DCAT event, we want to extend a warm invitation to all attendees. Axplora is committed to providing solutions in the ever-evolving landscape of the pharmaceutical industry by utilizing a diverse range of global manufacturing capabilities in over three continents and connecting dedicated scientists with cutting-edge technologies. Schedule a brief conversation with us to discuss how Axplora can contribute to your success and your patient success!"