HPAPI manufacturing

The demand for Highly Potent Active Pharmaceutical Ingredients (HPAPIs) is growing steadily, driven by the need for targeted medicines in the oncology field.
An HPAPI is usually defined as an API with an occupational exposure limit (OEL) at/or below 10 µg/m³ of air as an 8-h time-weighted average.
Due to the risks associated with their high pharmacological activity, the development and manufacturing of HPAPIs must ensure the safety of both patients and employees. This requires a strong quality system and HSE culture, and specialized high containment facilities operated by experienced personnel.
By nature, HPAPIs are often complex molecules and need specialized technologies for multi-step synthesis and purification.
Recognized expertise in HPAPI manufacturing
Axplora has a proven track record in the development and commercial manufacturing of HPAPIs with more than 300 commercial & clinical cGMP batches produced.
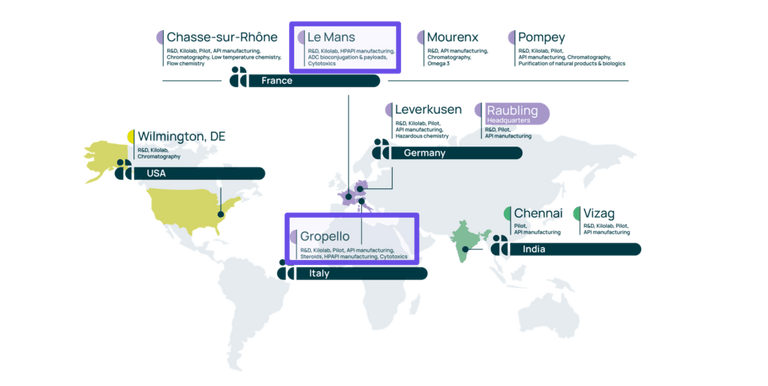
Our Le Mans site located in France, and our Gropello site in Italy, are centers of excellence for highly potent APIs. These sites offer more than 30 years’ experience in the manufacturing of HPAPIs & cytotoxic.
When our Le Mans site provides specific expertise in the field of ADC payloads, in addition to ADC conjugation services, our Gropello site offers services for steroids, cytotoxic, and other HPAPIs.
Learn more about our expertise in HPAPI manufacturing:
| Type of content | Title |
| Leaflet including a case study | Process robustness optimization and large-scale manufacture of a potent API, for the treatment of cancer from Phase III to commercial |
| Presentation recording | Safe HPAPI manufacturing: Thinking outside the glovebox! |
Handling of HPAPIs
Our Le Mans site can handle highly potent compounds with an OEL below 10 ng/m³/8hrs & cytotoxic with a maximum batch size of 10 kgs and our Gropello site can handle highly potent compounds with an OEL up to 10 ng/m³/8hrs.
Potent compounds, between 1-10 μg/m³/8hrs, can be manipulated at Axplora Gropello (Italy) and Chasse-sur-Rhône (France) with a batch size of up to 350 kgs.
We are able to handle a wide range of chemistry. Low and high-temperature reactions, as well as reduction or oxidation processes, can be run in our HPAPI facilities.

Our solutions for high-potency manufacturing
We offer a full range of high-potency manufacturing services, from process development to cGMP production:
- Process development
- Transfer and continuous improvement of established processes
- Development and scale-up of new processes
- Development, optimization, and validation of analytical methods
- Safety assessments
- Kilolabs
- cGMP production for clinical trials
- Process validation
- Commercial supply
- Large-scale production
- cGMP production for clinical trials
- Process validation
- Commercial supply
- Quality control
- Method development/transfer/validation
- IPC
- Release testing
- ICH Stability studies
- Regulatory support
Our centers of excellence for HPAPI manufacturing
Our two European sites involved in HPAPI production have been successfully inspected by the FDA & European authorities recently.
Axplora Le Mans site :

Axplora Gropello site:
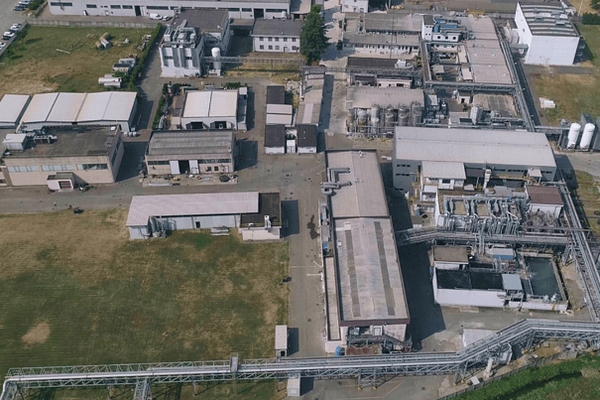
Our commitment to HPAPIs is demonstrated by our growing investments. We recently announced a €12M investment to extend HPAPI manufacturing capabilities at our Le Mans site.
